FDA Approves New IUD Designed To Be More Affordable
Liletta, an IUD just approved by the FDA, is being marketed in the United States through a unique partnership between manufacturers who hope to bring the device to more people at a lower cost. However, it is still unclear whether those savings will be felt by all women.
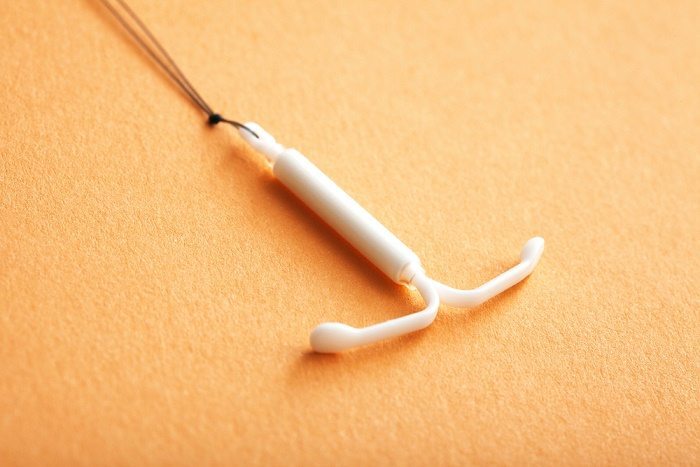
On March 2, the Food and Drug Administration (FDA) approved a new intrauterine device (IUD) that, among other things, was designed with affordability in mind. Liletta, which is already available in Europe, is being marketed in the United States through a unique partnership between manufacturers who hope to bring the device to more people at a lower cost. However, it is still unclear whether those savings will be felt by all women.
When released, Liletta will be the fourth IUD currently available in the United States. It joins ParaGard (also called the Copper-T), which began being sold to women in the United States in 1988; Mirena, a hormonal IUD introduced in 2000; and Skyla, a smaller version of that hormonal IUD which was released just last year with younger users in mind.
All IUDs are small T-shaped devices that are inserted into the uterus by a health-care professional and serve to block sperm from moving through the uterus toward the egg. ParaGard also releases copper, which some experts believe creates a substance toxic to sperm. In addition, Mirena, Skyla, and now Liletta all release levonorgestrel, a hormone similar to those found in birth control pills. This means that they also thicken cervical mucus (creating a barrier to sperm) and may suppress ovulation in some women. ParaGard lasts for ten years, Mirena for five, and Skyla and Liletta for three—but all can be removed by a health-care provider at any point if a woman decides she wants to become pregnant or switch methods.
Some of the primary benefits of IUDs are their ease of use and efficacy in preventing pregnancy. Unlike condoms, the pill, or other hormonal methods such as the patch or ring, once in place an IUD works for years without any thought or action on the part of the user. This doesn’t just take pressure off the user to remember their birth control; it takes user error out of the efficacy equation altogether. It’s not surprising, therefore, that IUDs have among the lowest failure rates of any contraceptive method. In clinical trials, Mirena had a failure rate of 0.2 percent; Skyla had a failure rate of 0.9 percent; and ParaGard had a failure rate ranging from 0.6 to 1.0 percent. Liletta has a failure rate of 0.55 percent. For comparison, the pill has similar failure rates under perfect conditions, but the possibility of user error makes the typical-use failure rate a much higher 9 percent.
The FDA approval of Liletta is based on a U.S. efficacy trial of 1,751 women, which the manufacturers say is the largest study of a hormonal method conducted in this country. The results of the study to date show that Liletta is 99.45 percent effective at preventing pregnancy for three years, and that it is safe for women regardless of their body mass index or whether or not they have had children. The study is ongoing, in part, to determine whether Liletta could continue working past its currently approved three-year lifespan.
Liletta’s manufacturers say that their partnership will make the device affordable to women of all income levels. One of the main complaints about IUDs today is their high cost. Between the price of the device itself and the provider’s fee for insertion, an IUD can cost as much as $1,000. While this is actually more cost-effective than other methods paid for on a monthly basis—a woman who uses ParaGard for its full ten-year lifespan, for example, could pay less than 30 cents a day—having to come up with the money up front is prohibitive for many women.
As Rewire recently reported, IUDs can even be difficult for Medicaid patients to access, as reimbursement rules in many states are problematic in different ways. In some states, for example, Medicaid reimbursement rates are less than the cost of the actual device, which means providers inevitably lose money. The high cost of the device also means that many providers and clinics can’t stock them in advance, so women who want IUDs will have to come back at least a second time for the insertion. Moreover, some states’ rules mean Medicaid will only pay for a device prescribed to a specific woman. This doesn’t just prevent providers from stocking them in advance of a patient request; it means if that specific patient does not return for insertion, the device can’t be used for anyone else. A number of states are working to fix this issue by loosening rules and increasing reimbursement.
Liletta may also be part of that solution, as it was designed with access in mind. Medicine 360, one of the companies marketing this new device, is a nonprofit pharmaceutical company. It will make Liletta available at a reduced price to clinics enrolled in the 340B Drug Pricing program. The 340B program is run by the U.S. Department of Health and Human Services and is available to entities that typically serve low-income women such as Title X family planning clinics, STD clinics, and Federally Qualified Health Centers. The exact pricing to these organizations will not be available until the device is actually released. It is likely—though not guaranteed—that savings will trickle down to patients who get their care at these locations.
However, for those obtaining Liletta through private providers, it is unclear whether Liletta will be cheaper than the three IUDs already on the market. Elle.com wrote that we should “expect it to be markedly more wallet-friendly than the existing IUDs on the market when it launches this spring.” But when Rewire reached out to Actavis, the Dublin-based pharmaceutical company that will be handling the commercial sales of Liletta, the company gave no specifics. In an email, a spokesperson said: “Actavis is committed to providing access to IUDs in the private sector. More information on patient programs will be provided when LILETTA is available.” She added: “Information about pricing will be provided when LILETTA is commercially available.”
After their near-extinction in the 1980s, IUDs continue to increase in popularity—the latest National Survey of Family Growth shows that 6.4 percent of women ages 15-to-44 are relying on this method of contraception. These numbers may very well go up when Liletta becomes available and helps at least some women overcome the price barrier to accessing this safe and effective form of contraception.
CORRECTION: This piece has been updated to reflect the correct daily price of an IUD when used for ten years.
